Giulio Natta and the Isotactic Polypropylene
A scientific and human profile of Prof. Natta
Giulio Natta, the only Italian to date awarded with the Nobel Prize for Chemistry, was born in 1903 in Porto Maurizio [1] in that western side of Italy where the "Ligurian Riviera" joins, once arrived along the sea on the French border, with the more famous "Côte d'Azur". A singular coincidence arranged that Natta was born a few kilometers from Sanremo, the Italian town where Alfred Nobel himself had lived for a long time and had died a few years earlier; Nobel is a character today certainly better known as a philanthropist but, in life, he had been a chemist and a first magnitude inventor. Also in Sanremo, where he often went on vacation, Prof. Natta received the news of the awarding of the Chemistry Prize by the Royal Academy of Sciences in Stockholm [1].
Immediately showing lively intelligence, the little child Giulio had learned to read, thanks to his mother Elena Crespi, from the age of 3. After his studies at the "Cristoforo Colombo" lyceum in Genoa, he enrolled at the age of only 16 (since the school system allowed it at the time) in the two-year preparatory course of mathematics, also in Genoa. Then he was admitted to the three-year university program in Industrial Engineering at the Polytechnic of Milan, in what would become and still is the “Department of Chemistry, Materials and Chemical Engineering” which is today named after him. Brilliant student, Natta graduated in engineering in 1924, without giving up an active social life. [1]
Among the very first chemical studies to which he devoted himself during his military service, the study on the effects of mustard gas should be noted. This is a toxic gas that was sadly known during the First World War. Mustard gas was known for its devastating effects on the eyes and lungs of soldiers who had encounter this substance. Tests that Natta performed on his skin, albeit with small amounts of Mustard gas, permanently marked his wrists. [1]
Since 1925 Natta received the assignment to teach Analytical Chemistry, a position he held until 1932. In 1935 he married Rosita Beati, a woman with a humanities education, who would later help him to name 'isotactic' and 'syndiotactic' (of Greek etymology) the different configurations of polymers that the professor discovered. In the second half of the 1930s the young scholar also taught in Rome and at the Polytechnic of Turin, but the nefarious approval of the "racial laws" in Italy, in 1938, ousted from the teaching position Prof. Mario Giacomo Levi (who was Jewish). These circumstances led Natta to the teaching of Industrial Chemistry at the Polytechnic of Milan, a commitment that he left only in 1973. [1]
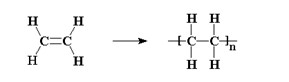
Natta was not what is said to be a 'specialist', he was a chemist with multifaceted knowledge and interests, but when in 1932 he had the opportunity to work in the laboratories in Freiburg (D) directed by the future Nobel Prize for chemistry Hermann Staudinger, the old master transmitted to him the enthusiasm for the study of macromolecules and polymers [1], that is, those substances made up of giant molecules (which to neophytes are often presented as a sort of long 'spaghetti') which are produced by chemically connecting in a repetitive sequence, simpler chemical units, the monomers.
It was mainly the passion for macromolecules that led Natta to the Nobel Prize and to the granting of over four thousand industrial patents filed between 1927 and 1974 [1]. But this did not mean that the professor became a specialist for this reason; on the contrary, he devoted himself also to activities that had nothing to do with macromolecules. For example, starting from the 1940s he studied the catalysts, mechanisms and kinetics of chemical reactions, with particular regard to the industrial production of methanol and formaldehyde. From these years he had scientific relations with various important Italian industrial companies, among which it is important to mention BPD at Colleferro and Montecatini (which later became Montedison in 1970); the latter would give to Natta's work a global importance.
The discovery of stereospecific polymerization, which is the heart of the Italian scientist's contribution to chemistry, has its roots in the works of Karl Ziegler in Muelheim (D), the German colleague who would eventually share with him the 1963 Nobel. Ziegler in 1952 studied the polymerization of ethylene in the presence of specific aluminium compounds (aluminium alkyls) but was unable to obtain high molecular weights products (i.e. polymer chains long enough to have a relevant industrial interest) until he added to the reagents titanium tetrachloride. Natta did not give to this German result a simply empirical value, he also understood that the titanium compound assumed in the reaction the function of a catalytic activator on ethylene, that is, it brought this substance into a state of greater aptitude to react. Without TiCl4, the reaction took place in one step, joining two ethylene molecules and simply forming its dimer, while with the addition of the tetrachloride the polymerization reaction started, which easily led to very long polyethylene chains.
Natta's intuition was that the activation of the monomer by the metal chloride catalyst had to be a process of more extensive importance, that is, it must also apply to the polymerization, for example, of propylene and other α-olefins. An intuition that turned out to be brilliant.
Stereospecific catalysts and isotactic polypropylene
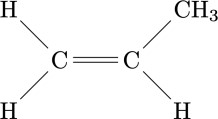
The adjective 'stereospecific' refers to the ability of a chemical process to generate reaction products with a structure that has a precise arrangement in space. This concept is not, however, easily understood as long as the reaction that generates a macromolecule involves highly symmetrical molecules. For example, ethylene has a highly symmetrical chemical structure: if a polymer is generated from ethylene, the individual monomeric units, even by rotating in space, have little chance of showing particularities. Not so with asymmetric or lower symmetry monomers: propylene, for example, has the methyl group CH3 which in the figure on the side is located at the top right; however, considering three-dimensional space and rotating along an axis in the plane of the paper and perpendicular to the double bond C = C, the same molecule can be found with the methyl group in the upper left, rather than in the other two available positions. As long as the molecule is considered, such arrangements are completely indifferent, but if a polymer chain is generated the geometry of what is obtained is strongly dependent on the way in which the single monomer units bind together. The ability of a catalytic substance (such as the aforementioned titanium tetrachloride) to mutually arrange the individual monomers in space in one way rather than another is called 'stereospecificity'. The first polymer that highlighted the existence of stereospecific polymerization catalysts was the polypropylene obtained by Giulio Natta and coworkers in Milan, on March 11, 1954 [2]. The discovery proved to be revolutionary for chemical industry worldwide.
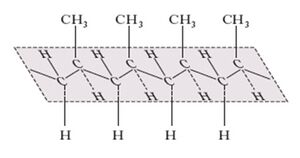
The more important polypropylene synthesized and isolated by Natta revealed to have a structure called, from then on, 'isotactic', that is, made up of a very regular chain of identical propylene units, all arranged in the same way (the head of a molecule linked to the tail of the next) with the CH3 groups all arranged on the same side with respect to the plane of the main chain assumed to adopt a planar zig-zag arrangement. However, Natta's studies also led to polymers with a different regularity: for example, the head-to-tail junction, with successive CH3 groups alternating on opposite sides of the main chain plane, leads to 'syndiotactic' polypropylene. The term ‘atactic’, was on the other hand adopted, to describe a polypropylene lacking a specific regularity in the arrangement of the CH3 groups.
It was soon recognized that the isotactic polymer had much better characteristics, in particular mechanical ones, than the atactic polymer: this circumstance amplified the importance of studies on catalysts capable of arranging in space, in a precise way, the atoms of specific molecules: the stereospecific catalysts.
The first patent on isotactic polypropylene was registered in Italy by Montecatini on June 8th, 1954, in the name of Giulio Natta, Piero Pino and Giorgio Mazzanti. In the following years the patent was then extended to many other countries, including the USA. [2]
Beyond the commercial aspect, however, the scientific community realized that Giulio Natta's team had interrupted Nature's monopoly with respect to the synthesis of stereoregular substances (that is, allowing to adopt a regular structure in space): already at the beginning of 1955 the American chemist Paul Flory (who was going to be awarded the Nobel Prize in Chemistry in 1974) wrote to Natta judging “of extraordinary interest and revolutionary significance” the manuscripts of the Italian professor. Natta at that time was already a member of the Accademia Nazionale dei Lincei (the scientific association of which Galileo Galilei had been part of starting from 1611).
In fact, as early as the 1950s, we knew very well about the existence of large molecules with an extremely regular structure, such as cellulose and some vegetal rubbers, but we had no idea how natural processes could achieve this result [2].
Natta had therefore successfully indicated a way forward for the synthesis of increasingly complex synthetic materials.
Modern developments of Natta's ideas: from cleaning buckets to rocket engines
One of the key points in Natta's work concerned the possibility of producing new crystalline macromolecules. Indeed very often structural regularity, in polymers but not only, is prerequisite for crystallization. The formation of crystals was immediately noticed by the team of the Italian scholar by observing isotactic polypropylene under the microscope and in X-ray diffraction experiments; it was recognized as a strong indication of structural regularity. The mechanical and technological properties of this polymer were also considered very interesting, from an industrial point of view, right from the first tests. Particularly suitable for use in household products were the tensile strength and temperature resistance of isotactic polypropylene.
To further realize, on a practical level, the significance of Natta's discovery, just think that even natural rubbers (which are typically isoprene polymers) exist in different types that have very different names and different mechanical-technological characteristics. For example, natural rubber and gutta-percha are closely related polymers, with the same composition albeit with different regular spatial organization of the atoms in the repeating unit. While the first is a rubber and hence in essence amorphous at room temperature, the latter is semicrystalline, however with properties that allow limited applications.

Stereospecific catalysts have therefore also revolutionized the rubber industry, making the extraction of latex from trees progressively less attractive, replacing the extraction with an industrial chemical process capable of reproducing it.
Finally, it is worth mentioning the reason why Natta's work can be placed, at least in part, in the wake of Alfred Nobel's research on the binders of energetic materials. The modern propellants of rocket engines, for example, were born in 1942 in California with the composition called Galcit-53 (studied by the propulsive research group of Von Karman, the founder of the AGARD group) which solved for the first time the age-old problem of keeping together, with a binder, the very heterogeneous materials that make up these propellants, using a petroleum derivative: the asphalt [4].
In the 2020s these rocket propellant mixtures did not change much: for the most part they consist of a substance with a high oxygen content (normally powdered ammonium perchlorate), a fuel (usually aluminum in micrometric powder) and a small percentage (10-15%) of a rubbery binder, usually a hydroxyl terminated polybutadiene (HTPB). The mechanical characteristics of the binder of a solid propellant are vital in the life of a rocket engine, because any cracking of the energy material during operation, at very high pressures and temperatures, almost always leads to the explosion of the motor. Therefore, also in this sector, that of the boosters of large space launchers such as the Ariane 5 system, but probably also in military applications, the use of stereoregular polymers with well defined chemical structure, obtained with very specific chemical processes of molecular ordering, is fundamental.
Carlo Buongiorno, first general director of the Italian Space Agency and Italian member of AGARD, was also referring to these topics when, in his autobiographical interview entitled "The space of a life", he cited Giulio Natta's discovery as being very important also for the development of the sector of space launchers. [5]
Author: Antonio di Biase - Politecnico di Milano (external fellow) - AIAA professional member.
References
1. Pasquon I. – “Giulio Natta”- Dizionario Biografico degli Italiani – Treccani.it - 2013
2. “Convegno su Giulio Natta a cento anni dalla nascita” – Accademia Nazionale dei Lincei – 2003
3. The entry “Polipropilene” in Enciclopedia Italiana – Treccani.it – (retrieved Aprile 2022)
4. De Luca L.T. – “GALCIT Projects: The Birth of US Rocketry” – NTREM 2017
5. Ferrone E. – “Carlo Buongiorno – Lo spazio di una vita” – LoGisma – 2011